On July 14, 2025, the Centers for Medicare & Medicaid Services (“CMS”) issued the CY 2026 Medicare Physician Fee Schedule (“PFS”) Proposed Rule. Published in the Federal Register on July 16, 2025, the rule outlines wide-ranging reforms to Medicare Part B payments, including substantive updates to remote patient monitoring (“RPM”) and remote therapeutic monitoring (“RTM”). (Federal Register Notice)
These proposals represent CMS’s most significant effort yet to recalibrate remote monitoring reimbursement. By lowering billing thresholds and introducing new code families, CMS aims to reduce operational barriers, expand clinical applicability, and reinforce its policy goal of shifting care delivery toward preventive and value-based models.
I. Proposed RPM and RTM Changes
A. Device Supply and Data Transmission
-
-
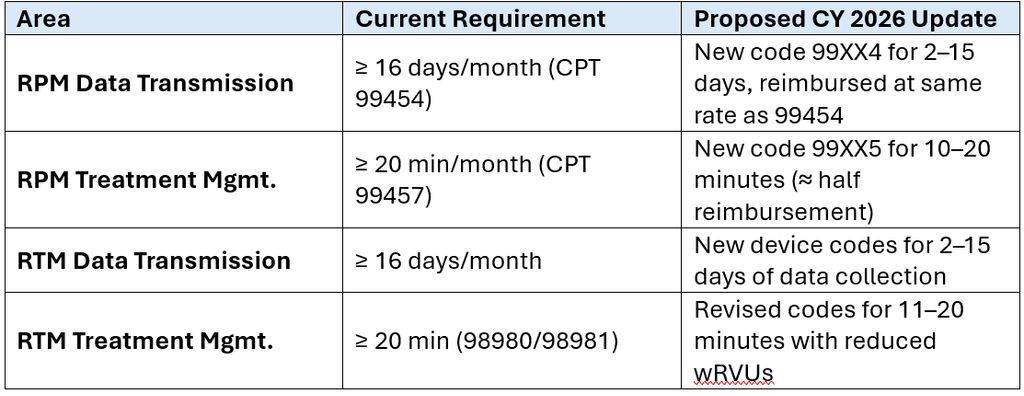
- Current Standard: At least 16 days of data transmission per 30-day period (CPT 99454).
- Proposed Revision: New code 99XX4 allows billing for 2–15 days of data transmission, reimbursed at the same rate as 99454.
-
B. Treatment Management Services
-
-
- Current Standard: ≥ 20 minutes of clinical interaction per month (CPT 99457).
- Proposed Revision: New code 99XX5 covers 10–20 minutes, with reimbursement set at approximately half the 99457 rate.
-
C. RTM Code Revisions
-
-
- Current Standard: ≥ 16 days of data and ≥ 20 minutes of interaction (CPT 98980/98981).
- Proposed Revision: New codes for 2–15 days of data and 11–20 minutes of interaction, with lower work relative value units (“wRVUs”) reflecting reduced workload.
-
II. Summary Table
III. Policy Rationale
CMS explained that these revisions are intended to modernize RPM and RTM utilization by aligning reimbursement with real-world practice. Many patients do not consistently generate 16 days of data each month, yet providers still perform meaningful clinical work in reviewing and managing limited data. By creating new pathways for reimbursement, CMS recognizes that remote monitoring can be clinically useful even when performed intermittently or for acute episodes.
The new code structure also reflects CMS’s valuation methodology. Rather than relying solely on historical physician work estimates, CMS is looking at actual resource inputs (clinical staff time, supplies, and equipment) and applying proportional reductions in wRVUs for shorter-duration services.
IV. Implications for Value-Based Care (“VBC”)
Remote monitoring has become an essential tool for providers engaged in value-based arrangements, including the Medicare Shared Savings Program (“MSSP”) and other alternative payment models. CMS’s proposed revisions have several direct implications for these models:
-
- Improved Attribution and Engagement:
By lowering the 16-day threshold, more beneficiaries can be included in RPM programs. This increases opportunities for early detection of complications, reduces hospital admissions, and supports the chronic disease management goals central to ACO contracts. - Integration with Preventive Care Metrics:
The ability to bill for shorter interactions (10–20 minutes) aligns with care coordination efforts measured under MSSP quality benchmarks. Providers can more accurately capture the time invested in outreach and management that previously went unreimbursed. - Support for Care Transition Episodes:
MSSP and other value-based entities place emphasis on reducing readmissions and managing post-acute episodes. The new codes create financial pathways to monitor patients intensively for a short period following hospital discharge, a use case not previously supported under the 16-day rule. - Alignment with MSSP Reform Initiatives:
CMS has also proposed changes to MSSP participation and benchmark methodologies in the CY 2026 PFS. Together with RPM flexibilities, these reforms provide practices and ACOs with expanded tools to manage populations under risk-bearing arrangements. (Federal Register Notice) - Operational Considerations:
Organizations engaged in VBC will need to ensure their RPM programs maintain strict compliance with device standards and documentation rules, especially given heightened DOJ enforcement. Expanded RPM billing opportunities, if improperly managed, could increase fraud and abuse risk in ways that may jeopardize both fee-for-service reimbursement and shared savings eligibility.
- Improved Attribution and Engagement:
V. Compliance and Oversight Context
Despite CMS’s efforts to broaden access, federal enforcement remains active. On June 26, 2025, the Department of Justice announced a $1.29 million False Claims Act settlement against Health Wealth Safe, Inc. and its owner, citing failures to provide FDA-approved devices, failing to provide devices that automatically report readings to the monitoring company without further human intervention, and violations of the Anti-Kickback Statute. (DOJ Press Release)
This case demonstrates the dual imperative: providers should embrace new RPM opportunities while implementing robust compliance frameworks to ensure adherence to CMS billing and supervision standards.
VI. Next Steps
The comment period for the CY 2026 PFS Proposed Rule closes on September 12, 2025. Stakeholders are encouraged to submit feedback via Regulations.gov. CMS will issue the final rule later this year, with changes scheduled to take effect on January 1, 2026.
VII. Conclusion
The CY 2026 PFS Proposed Rule marks a pivotal step in the evolution of RPM and RTM reimbursement. By loosening billing requirements, introducing new code families, and explicitly recognizing the value of shorter monitoring intervals, CMS has taken meaningful action to expand remote care. For providers engaged in value-based care, these changes present a clear opportunity to better integrate remote monitoring into population health strategies while securing appropriate reimbursement for time and resources expended.
At the same time, heightened enforcement activity highlights that compliance must remain a core priority. Organizations implementing RPM and RTM should invest in audit readiness, ensure device-based data collection, and align clinical documentation with CMS standards.
The Benesch Healthcare+ team is actively monitoring developments related to the CY 2026 PFS and MSSP reform and may provide additional updates as they become available. Please contact the authors of this article or another member of the Benesch Healthcare+ team for further information or guidance on how these proposed changes may affect your organization, including structuring compliant reimbursement models for both RPM and RTM.
Nesko Radovic at nradovic@beneschlaw.com or 312.506.3421.
Scott P. Downing at sdowning@beneschlaw.com or 312.624.6326.
Jason S. Greis at jgreis@beneschlaw.com or 312.624.6412.
Jake A. Cilek at jcilek@beneschlaw.com or 312.624.6363.
Christopher DeGrande at cdegrande@beneschlaw.com or 312.624.6364.
